Research Lines
Inicio Research
The main research line of CataNa is focused on Metal Nanoparticle Catalysis, but always from the perspective of organometallic chemistry. More specifically, it is centered on three main topics:
1) Influence of the Stabilizing Ligands in Metal Nanoparticle Catalysis.
During the last ten years, we have observed that an efficient strategy to control the activity and selectivity of a metal nanoparticle is the use of ancillary ligands that can transform the NP surface. As in organometallic complexes, surface ligands are able to modify the electronic and steric properties of MNPs, and therefore control their catalytic properties. Moreover, stabilizing ligands are also able to modulate other MNP properties such as morphology, solubility, stability or magnetic character. In this line, we have observed that MNPs can be stabilized with classic organometallic ligands (i. e. phosphines, N-hetrocyclic carbenes) or new specific ligands (i.e. imidazolium-amidinates) (Figure 1). As a whole, we are focused on the use of ancillary ligands as a new way to improve the selectivity and activity of the MNPs, approach until now mainly exploited by homogeneous catalysis (Acc. Chem. Res. 2018, 51, 376).
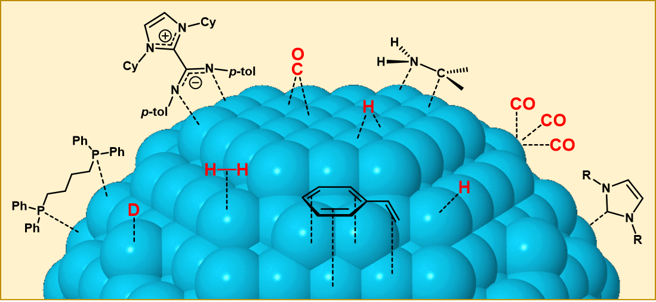
Figure 1. Coordination modes of stabilizing ligands and substrates on MNPs.
2) MNPs Supported on Graphene Materials.
In this research line we prepare graphene-supported MNPs by using an organometallic approach synthetic method. This consists in the decomposition of an organometallic precursor under mild conditions (r.t. and 3 bar H2) in the presence of a graphene support, which act as stabilizer (Figure 2). We have observed that the presence of heteroatoms in the graphene materials facilitates the generation of small, monodisperse and well distributed MNPs. Moreover, a clear influence of the support has been also observed on the activity and selectivity in hydrogenation reactions. For example, Ru NPs supported in N-doped graphene showed a remarkably activity and selectivity in the hydrogenation of palmitic acid to the corresponding fatty alcohol. In this case, the N-doped support not only stabilizes the Ru NPs, but it is also directly involved in the catalytic reactions (J. Catal. 2019, 377, 429).
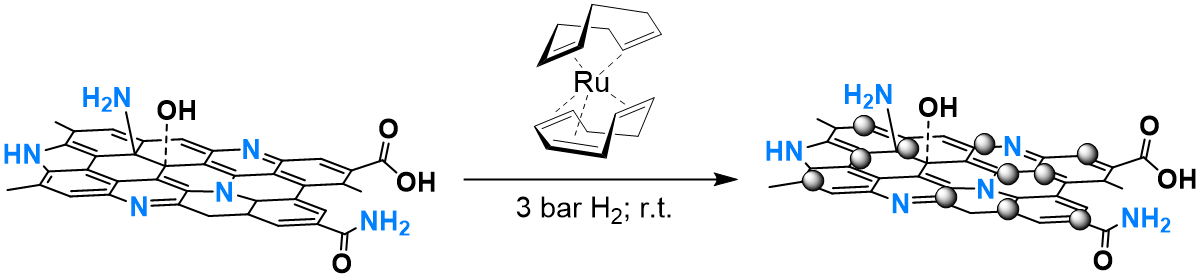
Figure 2. Synthesis of N-doped graphene-supported Ru NPs following the organometallic approach.
Following the same organometallic approach, we are also able to prepare bimetallic nanoparticles supported on N-doped graphene with different metal compositions. We observed that the activity and selectivity of the bimetallic NPs in the hydrogenation of acetophenone were highly dependent on the nanoparticle composition. Therefore, we were able to control the selectivity of these bimetallic nanocatalysts by adjusting their metal compositions (Catal Sci. Tech. 2021, 11, 494).
In addition, by this organometallic approach, we are also generating MNPs on nanographenes (Chem. Sci. 2022, 13, 13046) or reduced graphene oxides for hydrogen storage reactions (Catal Sci. Tech. 2022, 12, 1257) and transformation of biomass derived compounds (Nanoscale. 2022, 15, 12319).
3) Magnetically Induced Catalysis
Magnetic induction is an attractive alternative to conventional heating, which is generating great interest in the field of catalysis. This is based on the use of eddy currents and/or hysteresis losses produced in a ferromagnetic material by the presence of high-frequency alternating magnetic fields (AMF). The main advantage of magnetic heating Vs other heating techniques is its simplicity since this is a non-contact technique. Moreover, from an energetic point of view, it displays the highest power transmission since the energy is directly transferred inside the ferromagnetic material to be heated. All of this, combined with an extremely short warming time, make magnetic heating a very energetically efficient method.
This novel research line is centred in metal nanoparticles and magnetically induced catalysis. We have synthesized magnetically thermo-active magnetic nanoparticles (MagNPs) encapsulated in carbon (Figure 3), which apart to protect them from intense oxidation, it confers to the materials the stability necessary for high temperature reactions. These ultra-stable heating agents decorated with Ni or Pt-Sn, have demonstrated to be active in high Tª reactions such as, methanation, propane dry reforming and dehydrogenation of propane (ACS Appl. NanoMat. 2020, 3, 7076).
Encouraged by the efficient heating capacity of these MagNPs, we are currently synthetizing core@shell nanoparticles (Figure 3) also following an organometallic approach. Here the core is formed by FeCo NPs, which acts as heating agents, and the shell constitutes the catalytically active phase (Ni). These core@shell NPs are being employed in magnetically induced catalytic reduction of biomass-derived compounds in solution (ACS. Catal. 2022, 12, 8462).
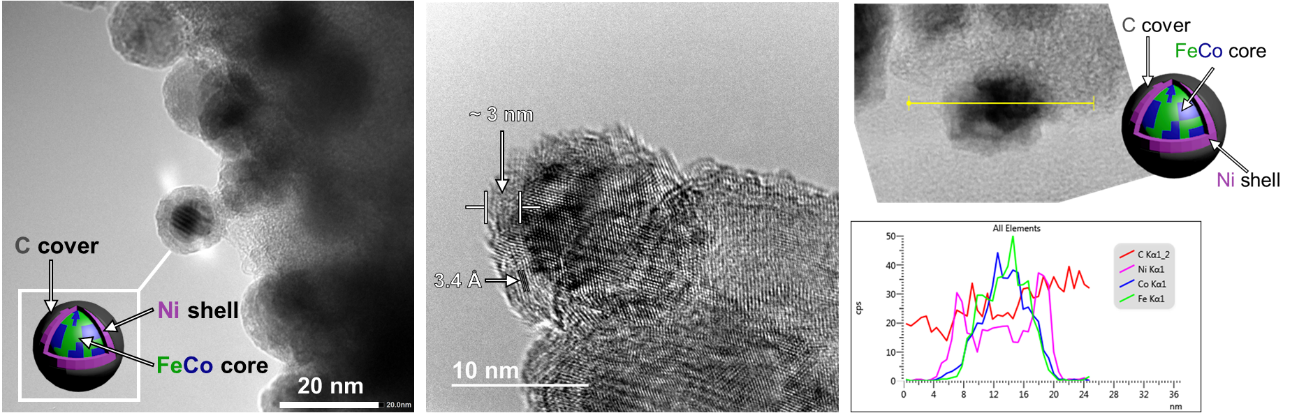
Figure 3. TEM images and EDX line-scan of FeCo@Ni NPs covered by carbon.
