Transposons are
segments of DNA that can move around to different positions in the genome of a
single cell. In the process, they may
- cause mutations
- increase (or decrease) the amount of DNA in the genome.
These mobile segments of DNA are sometimes called "jumping genes".
There are three distinct types:
- Class II Transposons consisting only of DNA that moves directly
from place to place.
- Class III Transposons; also known as Miniature
Inverted-repeats Transposable Elements or MITEs.
- Retrotransposons
(Class I) that
- first transcribe the DNA into RNA and then
- use reverse
transcriptase to make a DNA copy of the RNA to insert in a new location.
Many transposons
move by a "cut and paste" process: the transposon is cut out of its location
(like command/control-X on your computer) and inserted into a new
location (command/control-V).
This process requires an enzyme - a transposase - that is encoded
within some of these transposons.
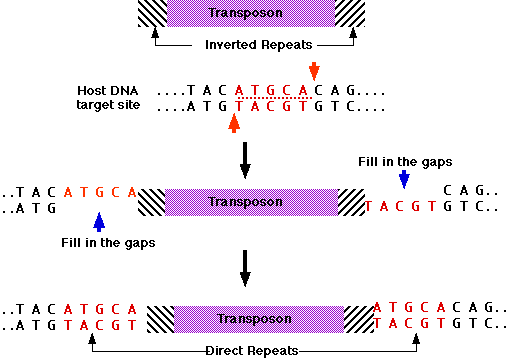 Transposase binds to:
Transposase binds to:
- both ends of the transposon, which consist of inverted repeats;
that is, identical sequences reading in opposite directions.
- a sequence of DNA that makes up the target site. Some transposases require
a specific sequence as their target site; other can insert the transposon
anywhere in the genome.
The DNA at the target site is cut in an offset manner (like the "sticky
ends"
produced by some restriction enzymes [Examples]).
After the transposon is ligated to the host DNA, the gaps are filled in by Watson-Crick
base pairing. This creates identical direct repeats at each end of
the transposon.
Often transposons lose their gene for transposase; but as long as somewhere
in the cell there is a transposon that can synthesize the enzyme, their inverted
repeats are recognized and they, too, can be moved to a new location.
The recent completion of the genome
sequence of rice and C.
elegans has revealed that their genomes contain thousands of copies of a
recurring motif consisting of
- almost identical sequences of about 400 base pairs flanked by
- characteristic inverted
repeats of about 15 base pairs such as
5' GGCCAGTCACAATGG..~400
nt..CCATTGTGACTGGCC 3'
3' CCGGTCAGTGTTACC..~400 nt..GGTAACACTGACCGG
5'
MITEs are too small to encode any protein. Just how they are
copied and moved to new locations is still uncertain. Probably larger
transposons that
- do encode the necessary enzyme and
- recognize the same inverted repeats
are responsible.
There are over 100,000 MITEs in the rice genome (representing some 6% of the
total genome). Some of the mutations found in certain strains of rice are caused
by insertion
of a MITE in the gene.
MITEs have also been found in the genome of humans, Xenopus, and apples.
The first
transposons were discovered in the 1940s by Barbara McClintock who worked with
maize (Zea mays, called "corn" in the U.S.). She found that they were
responsible for a variety of types of gene
mutations, usually 
- insertions
- deletions and
- translocations
| Some of the mutations (c, bz) used as examples of how
gene loci are mapped on the chromosome were caused by transposons. [Link] |
In developing somatic
tissues like corn
kernels, a mutation (e.g., c) that alters color will be passed on to
all the descendant cells. This produces the variegated pattern which is so
prized in "Indian corn". (Photo courtesy of Whalls Farms.)
It took about 40
years for other scientists to fully appreciate the significance of Barbara
McClintock's discoveries. She was finally awarded a Nobel Prize in 1983.
P elements are Class II transposons found in Drosophila. They
do little harm because expression of their transposase gene is usually repressed.
However, when male flies with P elements mate with female flies
lacking them, the transposase becomes active in the germline
producing so many mutations that their offspring are sterile.
In nature this is no longer a problem. P elements seem to have first appeared
in Drosophila melanogaster about 50 years ago. Since then, they have
spread through every population of the species. Today flies lacking P elements
can only be found in old strains maintained in the laboratory.
P elements have provided valuable tools for Drosophila geneticists. Transgenic
flies containing any desired gene can be produced by injecting the early
embryo with an engineered P element containing that gene.
Other transposons are being studied for their ability to create transgenic
insects of agricultural and public health importance.
Some
transposons in bacteria carry - in addition to the gene for transposase - genes
for one or more (usually more) proteins imparting resistance to antibiotics.
When such a transposon is incorporated in a plasmid,
it can leave the host cell and move to another. This is the way that the
alarming phenomenon of multidrug antibiotic
resistance spreads so rapidly.
Transposition in these cases occurs by a "copy (command/control-C) and
paste (command/control-V)" mechanism. This requires an additional enzyme - a
resolvase - that is also encoded in the transposon itself. The original
transposon remains at the original site while its copy is inserted at a new
site.
Retrotransposons move by a "copy and paste" mechanism but in contrast
to the transposons described above, the copy is made of RNA, not DNA.
The RNA copies are then transcribed back into DNA - using a reverse
transcriptase - and these are inserted into new locations in the genome.
Many retrotransposons have long terminal repeats (LTRs) at
their ends that may contain over 1000 base pairs in each.
Like DNA
transposons, retrotransposons generate direct repeats at their new sites of
insertion. In fact, it is the presence of these direct repeats that often is the
clue that the intervening stretch of DNA arrived there by retrotransposition.
About 40% of the entire human genome consists of retrotransposons.
HIV-1 - the cause of AIDS
- and other human retroviruses (e.g.,
HTLV-1, the human T-cell leukemia virus) behave like retrotransposons.
The RNA genome of HIV-1 contains a gene for
- reverse transcriptase and one for
- integrase. The integrase serves the same function as the
transposases of DNA transposons. The DNA copies can be inserted anywhere in
the genome.
Molecules of both enzymes are incorporated in the virus
particle.
The human genome contains over 500,000 LINEs (representing
some 16% of the genome). LINEs are long (~5,000 base pairs) DNA sequences that
represent reverse-transcribed RNA molecules originally transcribed by RNA
polymerase II; that is, messenger RNAs. Lacking introns as well as
the necessary control elements like promoters, these genes are not expressed.
They are called pseudogenes. However, some LINEs do encode a functional
reverse transcriptase and/or integrase. These enable them to
mobilize not only themselves but also
- other, otherwise nonfunctional, LINEs and
- Alu
sequences and other SINEs.
Because transposition is done by copy-paste, the number of LINEs can
increase in the genome. The diversity LINEs between individual human genomes
make them useful markers for DNA
"fingerprinting".
SINEs are short DNA sequences (100-500 base pairs) that
represent reverse-transcribed RNA molecules originally transcribed by RNA
polymerase III; that is, molecules of tRNA, 5S rRNA, and some other small
nuclear RNAs.
The most abundant SINEs are the Alu elements. There are about one
million copies in the human genome (representing about 11% of the total DNA).
Alu elements consist of a sequence of 300 base pairs containing a site that
is recognized by the restriction
enzyme AluI. They appear to be reverse transcripts of 7S RNA, part of the signal
recognition particle.
SINEs do not encode any functional molecules and (like LINEs) their presence
in the genome is a mystery. Like LINEs, they seem to represent only "junk" or
"selfish" DNA.
Transposons are mutagens. They can cause mutations in
several ways:
- If a transposon inserts itself into a functional gene, it will probably
damage it. Insertion into exons, introns, and even into DNA flanking the genes
(which may contain promoters
and enhancers)
can destroy or alter the gene's activity.
- Faulty repair of the gap left at the old site (in cut and paste
transposition) can lead to mutation there.
- The presence of a string of identical repeated sequences presents a
problem for precise pairing during meiosis. How is the third, say, of a string
of five Alu sequences on the "invading
strand" of one chromatid going to ensure that it pairs with the third
sequence in the other strand? If it accidentally pairs with one of the other
Alu sequences, the result will be an unequal crossover - one of the commonest
causes of duplications.
SINEs (mostly Alu sequences) and
LINEs cause only a small percentage of human mutations. (There may even be a
mechanism by which they avoid inserting themselves into functional genes.)
However, they have been found to be the cause of the mutations responsible for
some cases of human genetic diseases, including:
We don't know.
They have been called "junk" DNA and "selfish" DNA.
- "selfish" because their only function seems to make more copies of
themselves and
- "junk" because there is no obvious benefit to their host.
Because of the sequence similarities of all the LINEs and SINEs, they also
make up a large portion of the "repetitive DNA" of the cell.
Retrotransposons cannot be so selfish that they reduce the survival of their
host. Perhaps, they even confer some benefit. Two possibilities:
- Retrotransposons often carry some additional sequences at their 3' end as
they insert into a new location. Perhaps these occasionally create new
combinations of exons, promoters, and enhancers that benefit the host.
- Telomerase,
the enzyme essential for maintaining chromosome length, is closely related to
the reverse transcriptase of LINEs and may have evolved from it.
- Arabidopsis thaliana contains 1.3 x 108 base pairs (bp)
of DNA. This includes a small number of retrotransposons and probably about
25,000 functional genes.
- Maize (corn) contains almost 20 times more DNA (2.4 x 109 bp)
but surely has no need for 20 times as many genes. In fact, fully 50% of the
corn genome is made up of retrotransposons.
- Most of the 2.5 x 1011 bp of DNA in the genome of Psilotum
nudum is presumably "junk" DNA.
So it seems likely that the lack of an association between size of genome and
number of functional genes - the C-value
paradox - is caused by the amount of retrotransposon DNA accumulated in the
genome.
 Transposase binds to:
Transposase binds to:
